
Biongraft Barrier Membrane specifically engineered for periodontal restorative surgeries and assists in the regeneration of bone and periodontal support tissues. Fabricated from a biocompatible and bioresorbable medical grade poly(lactic acid) based synthetic polymer with a long history of safe medical use.
Product Characteristics
Fully resorbed
Fully resorbed after 15-20 weeks after implantation
Contains no tissue of human or animal origin
Contains no tissue of human or animal origin therefore carries no risk of disease transmission.
Prevents fibrous tissue growth
Prevents fibrous tissue growth in bone area.
No requirement for removing membranes
No requirement for removing membranes due to complete bioresorption.

Exterior side of Biongraft Dental Barrier Membrane compose of non-porous poly(lactic acid) (PLA) based film to prevent epithelial cells and fibroblast migration.
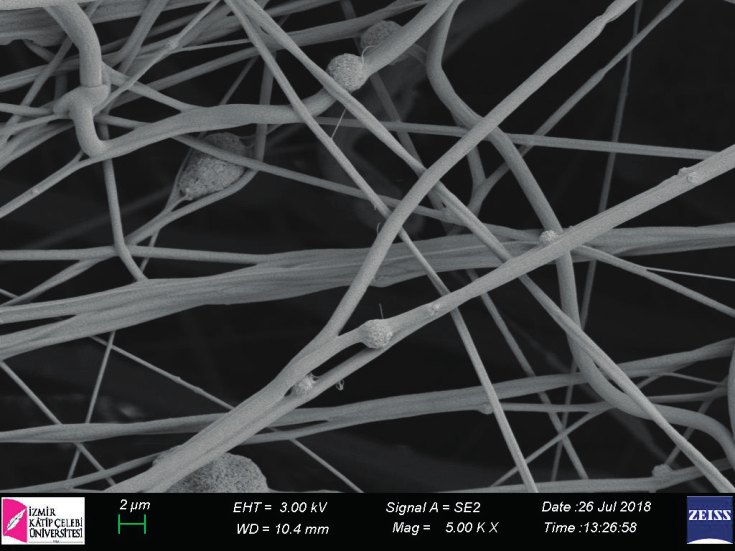
Interior side of Biongraft Dental Barrier Membrane compose of porous poly(lactic acid) (PLA) based microfibers to enhance mesenchymal stem cells adhesion, proliferation and differentiation.
Indications
- Ridge augmentation
- Lateral and Crestal Sinus Augmentation
- Sinus lifts
- Filling of defect of endodontic origin
- Cysts or other Osseous defects
- Alveolar bone regeneration

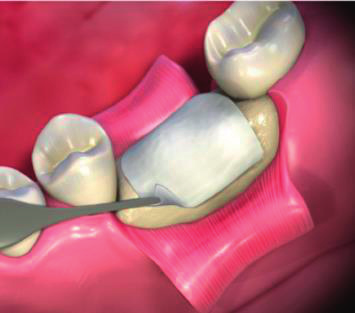
Application of Biongraft Dental Barrier Membrane.
Specifications

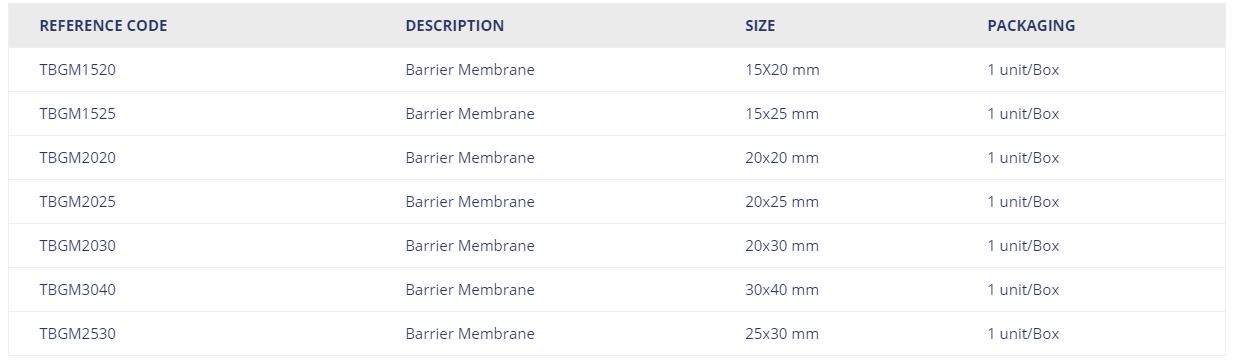
| REFERENCE CODE | DESCRIPTION | SIZE | PACKAGING |
| TBGM1520 | Barrier Membrane | 15X20 mm | 1 unit/Box |
| TBGM1525 | Barrier Membrane | 15×25 mm | 1 unit/Box |
| TBGM2020 | Barrier Membrane | 20×20 mm | 1 unit/Box |
| TBGM2025 | Barrier Membrane | 20×25 mm | 1 unit/Box |
| TBGM2030 | Barrier Membrane | 20×30 mm | 1 unit/Box |
| TBGM3040 | Barrier Membrane | 30×40 mm | 1 unit/Box |
| TBGM2530 | Barrier Membrane | 25×30 mm | 1 unit/Box |

ABOUT
Our mission is to acquire and commercialize innovative products for the Dental Sector.
